- 12.1. Clinical Trial
- 12.1.1. Central Nervous System
- 12.1.2. Head and Neck
- 12.1.2.1. Nasopharyngeal Carcinoma (NPC)
- 12.1.3. Bone
- 12.1.4. Haematology
- 12.1.5. Hepatobiliary
- 12.1.6. Tumour-Agnostic
- 12.2. Supportive Cancer Care
- 12.3. Translational Research
- 12.4. Basic Research
|
12.0 Paediatric |
||||||
|
12.1 Clinical Trial |
||||||
|
12.1.1.1 Central Nervous System (Neuroblastoma) |
||||||
|
Specific Selection Criteria: MYCN amplification |
||||||
|
Second-line treatment: Phase 2 |
Immunotherapy (Anti- Gd2 IGG3 Antibody) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
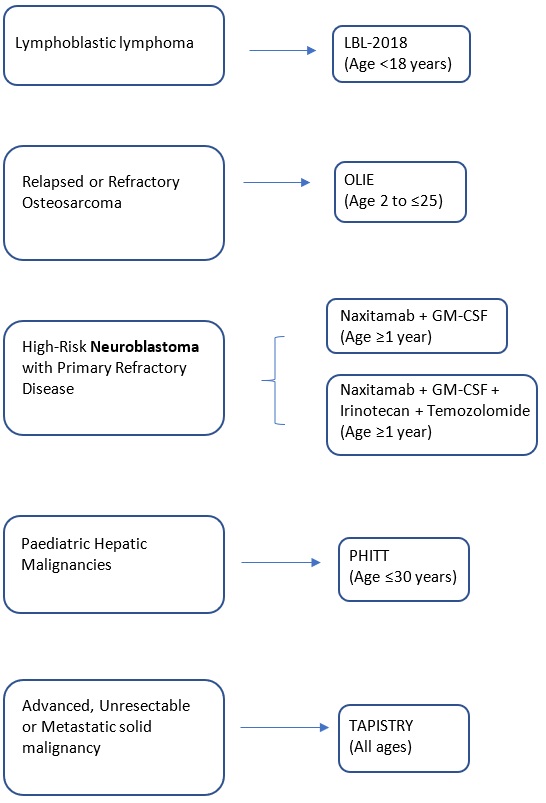
A Pivotal Phase 2 Trial of Antibody naxitamab (hu3F8) and Granulocyte-Macrophage Colony Stimulating Factor (GM- CSF) in High-Risk Neuroblastoma Patients with Primary Refractory Disease or Incomplete Response to Salvage Treatment in Bone and/or Bone Marrow. |
Key Inclusion criteria:
Key Exclusion criteria:
|
Naxitamab (Anti- Gd2 IGG3 Monoclonal Antibody) |
Professor Godfrey Chi-Fung Chan |
Department of Paediatrics & Adolescent Medicine |
gcfchan@hku.hk |
2255 4481 |
|
12.1.1.2 Central Nervous System (Neuroblastoma) |
||||||
|
Specific Selection Criteria: MYCN amplification |
||||||
|
Second-line treatment: Phase 2 |
Immunotherapy (Anti- Gd2 IGG3 Antibody), Granulocyte-Macrophage Colony Stimulating Factor, Chemotherapy |
|||||
|
An International, Single-Arm, Multicenter Phase 2 Trial. Naxitamab and Granulocyte-Macrophage Colony Stimulating Factor in Combination with Irinotecan and temozolomide in Patients with High-Risk Neuroblastoma with Primary Refractory Disease or in First Relapse. |
Key Inclusion criteria:
Key Exclusion criteria:
|
Naxitamab (Anti- Gd2 IGG3 Monoclonal Antibody) + Granulocyte-Macrophage Colony Stimulating Factor + Irinotecan (Chemo/ Topoisomerase I inhibitors) + temozolomide (Chemo) |
Professor Godfrey Chi-Fung Chan |
Department of Paediatrics & Adolescent Medicine |
gcfchan@hku.hk |
2255 4481 |
|
12.1 Clinical Trial |
||||||
|
12.1.3 Bone: Osteosarcoma |
||||||
|
Second-line or Third-line treatment: Phase 2 |
Targeted Therapy (Multiple Kinase Inhibitor), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
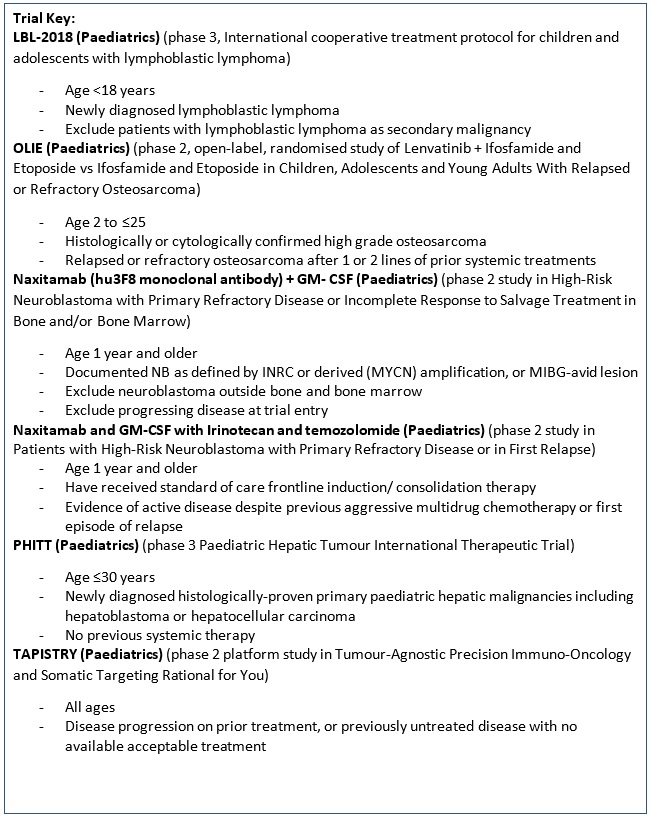
A Multicenter, Open-label, Randomized Phase 2 Study to Compare the Efficacy and Safety of Lenvatinib in Combination With Ifosfamide and Etoposide Versus Ifosfamide and Etoposide in Children, Adolescents and Young Adults With Relapsed or Refractory Osteosarcoma (OLIE). |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Lenvatinib (Multiple Kinase Inhibitor) + ifosfamide and etoposide
Vs.
Ifosfamide and etoposide |
Professor Godfrey Chi-Fung Chan |
Department of Paediatrics & Adolescent Medicine |
gcfchan@hku.hk |
Professor Godfrey Chi-Fung Chan 2255 4481 |
|
12.1 Clinical Trial |
||||||
|
12.1.4 Haematology (Lymphoblastic lymphoma) |
||||||
|
First-line treatment: Phase 3 |
Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
A Phase 3 trial, International cooperative treatment protocol for children and adolescents with lymphoblastic lymphoma. |
Key Inclusion Criteria:
Key Exclusion criteria:
|
Multiple treatment agents: Cyclophosphamide, Cytarabine, Dexamethasone, Daunorubicin, Doxorubicin, Ifosfamide, 6-Mercaptopurine, Methotrexate, PEG asparaginase, Prednisone, Prednisolone, Thioguanine, Vincristine, Vindesine. |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
|
12.1 Clinical Trial |
||||||
|
12.1.5 Hepatobiliary (Hepatic Malignancies) |
||||||
|
First-line treatment: Phase 3 |
Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
A Phase 3 trial, Pediatric Hepatic Tumor International Therapeutic Trial (PHITT). |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Multiple treatment agents: Carboplatin, Cisplatin, Doxorubicin, Etoposide, Fluorouracil, Gemcitabine, Irinotecan, Oxaliplatin, Sorafenib, Vincristine Sulfate. |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
|
12.1 Clinical Trial |
||||||
|
12.1.6 Tumour-Agnostic |
||||||
|
Advanced or Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: Multiple Oncogenic Genomic Alterations |
||||||
|
First-line or Second-line treatment: Phase 2 |
Immunotherapy / Targeted Therapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
Tumor-Agnostic Precision Immuno-Oncology and Somatic Targeting Rational for You (TAPISTRY) Phase II Platform Trial |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Entrectinib, Alectinib, Atezolizumab, Ipatasertib, Trastuzumab emtansine, Idasanutlin, Inavolisib, Belvarafenib, Pralsetinib, GDC-6036, Camonsertib.
|
Professor Godfrey Chi-Fung Chan |
Department of Paediatrics & Adolescent Medicine |
gcfchan@hku.hk |
2255 4481 |
|
12.0 Paediatric |
||||||
|
12.2 Supportive Cancer Care |
||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|
Individualized Neurofeedback treatment for paediatric brain tumour survivors with attentional deficits, a randomized-controlled trial (RCT) |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Winnie Wan-Yee Tso |
Department of Paediatrics & Adolescent Medicine |
wytso@hku.hk |
2255 4269 |
|
|
12.0 Paediatric |
||||||
|
12.3.1 Translational Research |
||||||
|
Study Title |
Principal Investigator |
Department |
|
Contact number |
||
|
HMRF - Longitudinal analysis of Epstein-Barr virus-specific T cells and NK cell responses in paediatric patients with infectious mononucleosis and post-transplant lymphoproliferative disorder - Laboratory research study on longitudinal patients' blood and plasma samples |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
||
|
12.3.2 Translational Research |
||||||
|
HMRF - Analysis of Epstein-Barr virus (EBV) genomic variations in EBV-associated lymphoproliferative diseases - Laboratory research study on next generation sequencing of viral genomes harbored in blood, saliva and plasma samples of patients |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
||
|
12.3.3 Translational Research |
||||||
|
HMRF - Characterisation of pathogenetic mechanisms of Epstein-Barr virus-associated T and natural killer cell lymphoproliferative diseases in children - Laboratory research study on immunology and genomics of patients' blood and plasma samples |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
||
|
12.0 Paediatric |
||||||
|
12.4.1 Basic Research (Brain Tumor) |
||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|
Exploring Neuro-electrophysiological Markers of Neurocognitive Outcomes in Paediatric Brain Tumour Survivors |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Winnie Wan-Yee Tso |
Department of Paediatrics & Adolescent Medicine |
wytso@hku.hk |
2255 4269 |
|
|
12.4.2 Basic Research (Brain Tumor and extra-CNS solid tumors) |
||||||
|
Multi-omic profiling of tumor tissue and liquid biome of pediatric tumors |
Key Inclusion Criteria:
|
Dr Anthony Pak-yin Liu |
Department of Paediatrics & Adolescent Medicine |
apyliu@hku.hk |
2255 4806 |
|
|
12.4.3 Basic Research (Nasopharyngeal Carcinoma) |
||||||
|
RGC - Targeting Epstein-Barr Virus in Nasopharyngeal Carcinoma: From Mechanistic Study to Novel Therapeutic Development - Multi-institutional basic science study. |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
||
|
12.4.4 Basic Research |
||||||
|
HMRF - Effects on induction of viral lytic cycle in Epstein-Barr virus-associated epithelial malignancies by combining histone deacetylase inhibitor and a novel organic compound grant - Laboratory research study on cell lines and animal models. |
Dr Alan Kwok-shing Chiang |
Department of Paediatrics & Adolescent Medicine |
chiangak@hku.hk |
2255 4482 |
||

Follow HKUMed