

- 4.1. Clinical Trial
- 4.1.1. Non-Small Cell Lung Cancer (NSCLC)
- 4.1.1.1. Oncogenic Driver Mutation
- 4.1.1.1.1. EGFR mutation
- 4.1.1.1.2. ALK
- 4.1.1.1.3. ROS1
- 4.1.1.1.4. KRAS
- 4.1.1.1.5. HER2
- 4.1.1.1.6. MET
- 4.1.1.2. Wildtype
- 4.1.1.2.1. PD-L1 High (≥50%)
- 4.1.1.2.2. PD-L1 Low (1-49%)
- 4.1.1.2.3. PD-L1 ≥1%
- 4.1.1.2.4. PD-L1 all comers
- 4.1.1.1. Oncogenic Driver Mutation
- 4.1.2. Small Cell Lung Cancer (SCLC)
- 4.1.1. Non-Small Cell Lung Cancer (NSCLC)
- 4.2. Supportive Cancer Care
- 4.3. Data Science
- 4.4. Translational Research
- 4.5. Basic Research
|
4.0 Lung |
||||||
|
4.1 Clinical Trial |
||||||
|
4.1.1 Non-Small Cell Lung Cancer (NSCLC) |
||||||
|
4.1.1.1 Oncogenic Driver Mutation |
||||||
|
4.1.1.1.1.1 EGFR mutation |
||||||
|
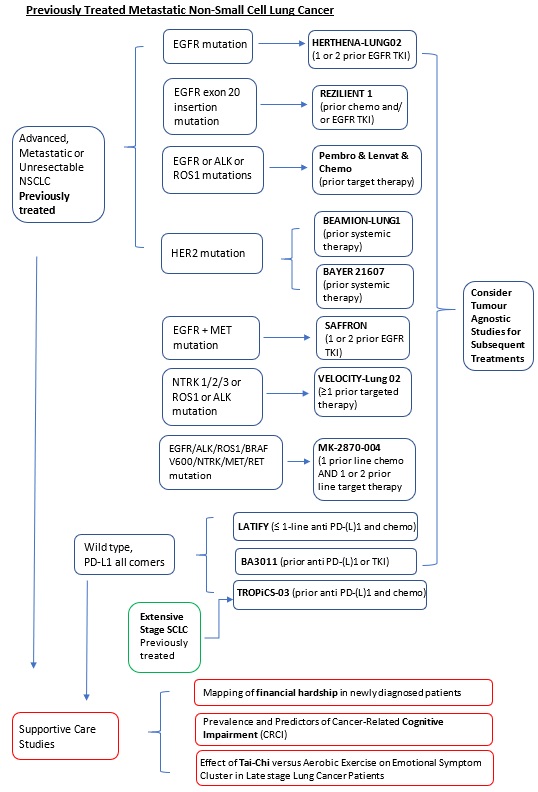
Locally Advanced or Metastatic |
||||||
|
Second-line or Subsequent treatment (Previously treated, failure to EGFR-TKI): Phase 3 |
Targeted Therapy (Anti-HER3 ADC) |
|||||
|
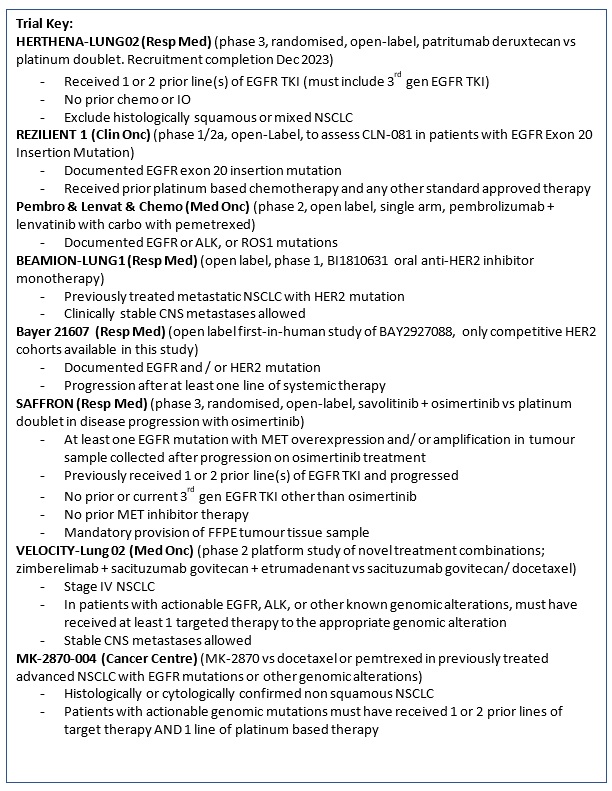
HERTHENA-Lung02 (Protocol ID: U31402-A-U301): A phase 3, randomized, open-label study of patritumab deruxtecan versus platinum-based chemotherapy in metastatic or locally advanced EGFR-mutated NSCLC after failure of EGFR tyrosine kinase inhibitor (TKI) therapy.
(This study expects recruitment completion in Nov/Dec 2023). |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Patritumab Deruxtecan (U3-1402; HER3-DXd) (Anti-HER3 Antibody Drug Conjugate)
Vs.
Carbo/Cis-platin + Pemetrexed (Chemo) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1.1.2 EGFR, MET mutation |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second or Third-line treatment: Phase 3 |
Targeted therapy (EGFR/ MET Inhibitor) |
|||||
|
SAFFRON (Protocol ID: D5087C00001): A phase 3, randomized, open-label study of savolitinib in combination with osimertinib versus platinum-based doublet chemotherapy in participants with EGFR mutated MET-positive, locally advanced or metastatic non-small cell lung cancer who have progressed following treatment with osimertinib. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Osimertinib (EGFR inhibitor) + savolitinib (MET inhibitor)
Vs.
Carbo/Cis-platin + Pemetrexed (Chemo) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1.1.3 EGFR mutation |
||||||
|
Early and Locally Advanced (Stage II – Stage IIIB), Resectable |
||||||
|
Adjuvant treatment: Phase 2 |
Targeted Therapy (EGFR inhibitor) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
TARGET (Protocol ID: D5162C00048): An open-label, single-arm, phase 2, multinational, multicentre study to assess the efficacy and safety of 5 years of osimertinib in participants with epidermal growth factor receptor mutation-positive stage II-IIIB non-small cell lung carcinoma, following complete tumour resection with or without adjuvant chemotherapy. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Osimertinib (EGFR inhibitor) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1.1.4 EGFR mutation |
||||||
|
Locally Advanced or Metastatic |
||||||
|
First-line treatment: Phase 2 |
Targeted Therapy (EGFR inhibitor/ EGFR+MET antibody) |
|||||
|
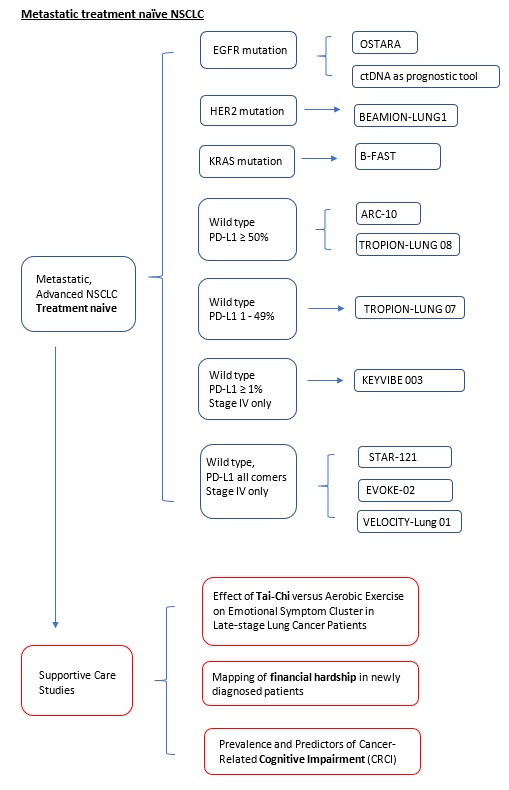
OSTARA (Protocol ID: D5162C00052): A phase 2, open-label, single-arm, multi-centre study to evaluate the safety and efficacy of osimertinib with amivantamab as first-line treatment in participants with epidermal growth factor receptor mutation-positive, locally advanced or metastatic Non-Small-Cell Lung Cancer.
(Wave 1 cohort will be ended soon. The "wave 2" cohort will be opened in early 2024) |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Osimertinib (EGFR inhibitor) (oral) + amivantamab (bispecific anti-EGFR + MET antibody) (IV) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1.1.5 EGFR, ALK, or ROS1 mutation |
||||||
|
Metastatic (Stage IV) |
||||||
|
Second-line treatment: Phase 2 |
Immunotherapy (Anti-PD-1), Targeted therapy (VEGFR inhibitor), Chemotherapy |
|||||
|
A Phase 2 Open-label Single-arm Study to Evaluate the Combination of pembrolizumab, lenvatinib and Chemotherapy in Non-small Cell Lung Cancer (NSCLC) Harbouring Targetable Mutation and Failed Standard Tyrosine Kinase Inhibitors. |
Key Inclusion Criteria:
|
Pembrolizumab + lenvatinib (VEGFR Inhibitor) + Pemetrexed + Carboplatin |
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
|
4.1.1.1.1.6 EGFR mutation |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second-line or Subsequent-line treatment (Previously treated, failure to platinum based chemotherapy and/or EGFR-TKIs): Phase 1/2a |
Targeted therapy (EGFR inhibitor) |
|||||
|
REZILIENT1 (Protocol ID: CLN-081-001): A Phase 1/2a, Open-Label, Multi-Center Trial to Assess Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of CLN-081 in Patients With Non-Small Cell Lung Cancer Harboring EGFR Exon 20 Insertion Mutations |
Key Inclusion Criteria:
|
CLN-081 (EGFR Inhibitor) |
Dr. Victor Lee |
Department of Clinical Oncology
|
vhflee@hku.hk |
Mike LAW 2255 5124 |
|
4.1.1.1 Oncogenic Driver Mutation |
||||||
|
4.1.1.1.4.1 KRAS mutation |
||||||
|
Advanced or Metastatic |
||||||
|
Second-line treatment: Phase 2/3 |
Targeted therapy (KRAS G12C inhibitor) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
B-FAST (Blood-First Assay Screening Trial, Protocol ID: BO29554, Cohort G): A Phase II/III Multicenter Study Evaluating the Efficacy and Safety of Multiple Targeted Therapies as Treatments for Patients With Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) Harboring Actionable Somatic Mutations Detected in Blood. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
GDC-6036 (KRAS G12C Inhibitor: Divarasib)
Vs.
Docetaxel (Chemo) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1 Oncogenic Driver Mutation |
||||||
|
4.1.1.1.5.1 HER2 mutation |
||||||
|
Advanced or Metastatic |
||||||
|
First or Subsequent-line treatment: Phase 1 |
Targeted Therapy (HER2 TKI Inhibitor) |
|||||
|
BEAMION-Lung1 (Protocol ID: 1479-0001): An open label, phase 1 dose escalation trial, with dose confirmation and expansion, of BI1810631 as monotherapy in patients with advanced or metastatic solid tumours with HER2 aberrations. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
BI 1810631 (Anti HER-2 inhibitor) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1.5.2 HER2 mutation and/or EGFR |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second-line or Subsequent-line treatment: Phase 1 |
Targeted therapy (EGFR/ HER2 inhibitor) |
|||||
|
BAYER21607 (Protocol ID: 21607): An open label, first-in-human study of BAY2927088 in participants with advanced non-small cell lung cancer (NSCLC) harboring an EGFR and/or HER2 mutation.
(only competitive HER2 cohorts available in this study) |
Key Inclusion Criteria:
|
BAY2927088 (EGFR and HER2 Inhibitor) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.1 Oncogenic Driver Mutation |
||||||
|
4.1.1.1.6.1 MET, EGFR mutation (same as study: 4.1.1.1.1.3) |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second or Third-line treatment: Phase 3 |
Targeted therapy (EGFR/ MET Inhibitor) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
SAFFRON (Protocol ID: D5087C00001): A phase 3, randomized, open-label study of savolitinib in combination with osimertinib versus platinum-based doublet chemotherapy in participants with EGFR mutated MET-positive, locally advanced or metastatic non-small cell lung cancer who have progressed following treatment with osimertinib. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Osimertinib (EGFR inhibitor) + savolitinib (MET inhibitor)
Vs.
Carbo/Cis-platin + Pemetrexed (Chemo) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1 NSCLC |
||||||
|
4.1.1.2 Wildtype |
||||||
|
4.1.1.2.1.1 PD-L1 High (≥50%) |
||||||
|
Locally Advanced or Metastatic |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-1 Antibody), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
ARC-10 (Protocol ID: 2020-003562-39): A Phase 3 Study to Evaluate Zimberelimab (AB122) Combined with Domvanalimab (AB154) Conpared to Pembrolizumab in Front-Line, PD-L1-Positive, Locally Advanced or Metastatic Non-Small Cell Lung Cancer. |
Key Inclusion Criteria:
|
Zimberelimab (AB122) + Domvanalimab (AB154)
vs
Pembrolizumab |
Dr. Victor Lee |
Department of Clinical Oncology
|
vhflee@hku.hk |
Angela IU 2255 5124 |
|
4.1.1 NSCLC |
||||||
|
4.1.1.2 Wildtype |
||||||
|
4.1.1.2.1.2 PD-L1 High (≥50%) |
||||||
|
Advanced or Metastatic, Unresectable |
||||||
|
First-line treatment: Phase 3 |
Targeted Therapy (Anti-Trop-2 ADC), Immunotherapy (Anti PD-1 antibody) |
|||||
|
Tropion-Lung08 A Randomized, Open-label, Phase 3 Trial of Dato-DXd Plus Pembrolizumab vs Pembrolizumab Alone in Treatment-naïve Subjects with Advanced or Metastatic PD-L1 High (TPS ≥50%) Non-small Cell Lung Cancer Without Actionable Genomic Alterations |
Key Inclusion Criteria
|
Pembrolizumab (Anti-PD-1 antibody) + Dato-DXd (Anti-Trop-2 ADC)
Vs
Pembrolizumab |
Dr. Victor LEE |
Department of Clinical Oncology
|
vhflee@hku.hk |
Angela IU 2255 5124 |
|
4.1.1 NSCLC |
||||||
|
4.1.1.2 Wildtype |
||||||
|
4.1.1.2.2.1 PD-L1 Low (1-49%) |
||||||
|
Advanced or Metastatic, Unresectable |
||||||
|
First-line treatment: Phase 3 |
Targeted Therapy (Anti-Trop-2 ADC), Immunotherapy (Anti-PD-1 antibody), Chemotherapy |
|||||
|
TROPION-Lung07 A Randomized Phase 3 Study of Datopotamab Deruxtecan (Dato-DXd) and Pembrolizumab, with or Without Platinum Chemotherapy, in Subjects with No Prior Therapy for Advanced or Metastatic PD-L1 TPS <50% Non-squamous Non-small Cell Lung Cancer Without Actionable Genomic Alterations |
Key Inclusion Criteria
|
Dao-DXd (Anti-Trop-2 ADC) + Pembrolizumab (Anti-PD-1 antibody) + Carboplatin (Chemotherapy)
Vs
Dato-DXd + Pembrolizumab
Vs
Pembrolizumab + Pemetrexed (Chemotherapy) + Carboplatin |
Dr. Victor LEE |
Department of Clinical Oncology
|
vhflee@hku.hk |
Angela IU 2255 5124 |
|
4.1.1 NSCLC |
||||||
|
4.1.1.2 Wildtype |
||||||
|
4.1.1.2.3.1 PD-L1 ≥1% |
||||||
|
Metastatic (Stage IV) |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-TIGIT antibody, Anti-PD-1 antibody) |
|||||
|
KEYVIBE-003 (Protocol ID: MK-7684A-003): A Phase 3, Multicenter, Randomized, Double-Blind Study of MK-7684 with pembrolizumab as a Coformulation (MK-7684A) Versus pembrolizumab Monotherapy as First Line Treatment for Participants With PD-L1 Positive Metastatic Non-Small Cell Lung Cancer. |
Key Inclusion Criteria:
|
MK-7684A (Vibostolimab (Anti-TIGIT Monoclonal antibody) + pembrolizumab (Anti-PD-1 monoclonal antibody)) |
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
|
4.1.1 NSCLC |
||||||
|
4.1.1.2 Wildtype |
||||||
|
4.1.1.2.4.1 PD-L1 all comers |
||||||
|
Metastatic (Stage IV) |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-1/ Anti-TIGIT Antibody), Chemotherapy |
|||||
|
STAR-121 (Protocol ID: GS-US-626-6216): A randomized, open-label, phase 3 study to evaluate zimberelimab and domvanalimab in combination with chemotherapy versus pembrolizumab with chemotherapy for the first-line treatment of patients with metastatic non-small cell lung cancer with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Zimberelimab (Anti-PD-1 Antibody) and domvanalimab (Anti-TIGIT Antibody) + Chemotherapy (Carbo/cisplatin + Pemetrexed/Paclitaxel)
Vs.
Zimberelimab (Anti-PD-1 Antibody) + Chemo-therapy
Vs.
Pembrolizumab (Anti-PD-1 Antibody) + Chemotherapy |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.2.4.2 PD-L1 all comers (same study as 4.1.1.2.4.1) |
||||||
|
Metastatic (Stage IV) |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-1/ Anti-TIGIT Antibody), Chemotherapy |
|||||
|
STAR-121 (Protocol ID: GS-US-626-6216): A randomized, open-label, phase 3 study to evaluate zimberelimab and domvanalimab in combination with chemotherapy versus pembrolizumab with chemotherapy for the first-line treatment of patients with metastatic non-small cell lung cancer with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Zimberelimab (Anti-PD-1 Antibody) and domvanalimab (Anti-TIGIT Antibody) + Chemotherapy (Carbo/cisplatin + Pemetrexed/Paclitaxel)
Vs.
Zimberelimab (Anti-PD-1 Antibody) + Chemo-therapy
Vs.
Pembrolizumab (Anti-PD-1 Antibody) + Chemotherapy |
Dr. Victor LEE |
Department of Clinical Oncology
|
vhflee@hku.hk |
Angela IU 2255 5124 |
|
4.1.1.2.4.3 PD-L1 all comers |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second-line or Third-line treatment: Phase 3 |
Targeted therapy (ATR Kinase Inhibitor), Immunotherapy (Anti-PD-L1 Antibody), Chemotherapy |
|||||
|
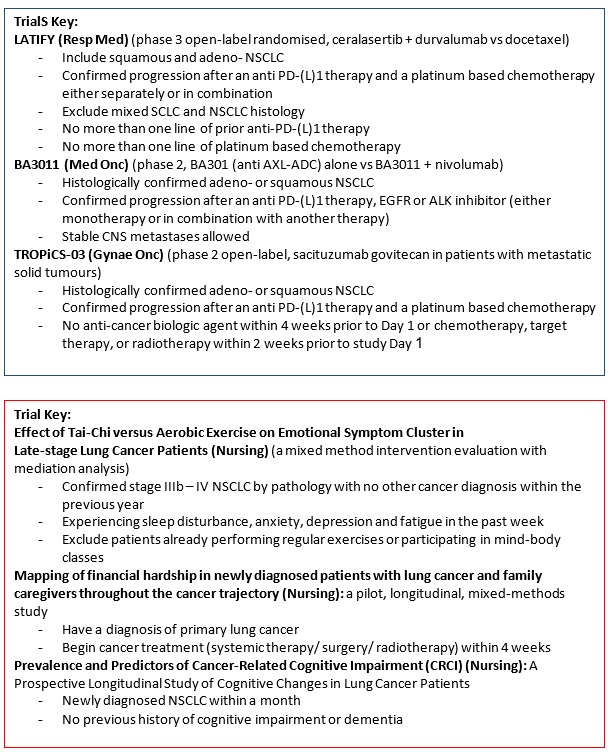
LATIFY (Protocol ID: D533BC00001): A phase 3, open-label, randomized, multicenter study of Ceralasertib plus durvalumab versus docetaxel in patients with advanced or metastatic non-small cell lung cancer whose disease has progressed on or after prior anti-PD-(L)1 therapy and platinum-based chemotherapy |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Ceralasertib (Oral) (ATR Kinase Inhibitor) + durvalumab (IV) (Anti-PD-L1 Antibody)
Vs.
Docetaxel (Chemo) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
|
4.1.1.2.4.4 PD-L1 all comers |
||||||
|
Metastatic (Stage IV) |
||||||
|
First-line treatment: Phase 2 |
Targeted Therapy (Anti-Trop2-ADC) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
EVOKE-02 (Protocol ID: GS-US-576-6220): An Open-label, Multicenter, Phase 2 Study of Sacituzumab Govitecan Combinations in First-line Treatment of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) Without Actionable Genomic Alterations. |
Key Inclusion Criteria:
|
Sacituzumab Govitecan-hziy (Antibody Drug Conjugate) + Pembrolizumab (Anti-PD-1 monoclonal antibody)
Vs.
SG + Pembrolizumab + Carboplatin(Chemo)
Vs.
SG + Pembrolizumab + Cisplatin |
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
|
4.1.1.2.4.5 PD-L1 all comers |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second-line or Subsequent-line treatment: Phase 2 |
Targeted therapy (Anti-AXL ADC), Immunotherapy (PD-1 inhibitor) |
|||||
|
A phase 2 study of BA3011 alone and in combination with PD-1 inhibitor in adult patients with metastatic non-small cell lung cancer (NSCLC) who had prior disease progression on a PD-1/L-1 inhibitor. |
Key Inclusion Criteria:
|
BA3011 (Antibody-drug conjugate (ADC))
Vs.
BA3011 (Antibody-drug conjugate (ADC)) + PD-1 inhibitor |
Dr. Thomas Yau |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
4.1.1.2.4.6 PD-L1 all comers |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Second-line or Subsequent-line treatment: Phase 2 |
Targeted Therapy: (Anti-HRS7-SN38 ADC) |
|||||
|
TROPiCS-03 (Protocol ID: IMMU-132-11): A phase 2, open-label study of sacituzumab govitecan (IMMU-132) in subjects with metastatic solid tumours. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Sacituzumab Govitecan (Anti-HRS7-SN38 ADC) |
Dr. Roland Leung |
Department of Obstetrics & Gynaecology |
tseky@hku.hk |
Dr. Ka Yu Tse 2255 5102 9061 9609 |
|
4.1.1.2.4.7 PD-L1 all comers |
||||||
|
Metastatic |
||||||
|
Phase 2 Substudy 01: First-line treatment Substudy 02: Second or Subsequent-line treatment for genomic alterations |
Immunotherapy: (Anti-PD-1/ Anti-TIGIT antibody), Targeted Therapy: (Anti-Trop-2 ADC/ Adenosine receptor antagonist), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
Velocity 01/02 (Protocol ID: GS-US-624-6376): A Phase 2 Platform Study Evaluating the Safety and Efficacy of Novel Treatment Combinations in Patients With Lung Cancer. |
Key Inclusion Criteria:
Substudy 01:
Substudy 02:
|
Substudy 01: zimberelimab (Anti-PD-1 antibody) + sacituzumab govitecan (Anti-Trop-2 ADC) + domvanalimab (Anti-TIGIT antibody)
Or
zimberelimab + domvanalimab + etrumadenant (dual A2A/A2B adenosine receptor antagonist)
Or
Zimberelimab + Etrumadenant
Vs
Zimberelimab + Platinum Based Chemotherapy
Substudy 02: zimberelimab + sacituzumab govitecan + Etrumadenant
Vs
sacituzumab govitecan / Docetaxel (Chemotherapy) |
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
4.0 Lung |
||||||
|
4.1 Clinical Trial |
||||||
|
4.1.2 SCLC |
||||||
|
Locally Advanced or Metastatic |
||||||
|
Specific Selection Criteria: Progressed after prior platinum-based chemotherapy and PD-(L)1 directed therapy |
||||||
|
Second-line treatment: Phase 2 |
Targeted Therapy: (Anti-HRS7-SN38 ADC) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
TROPiCS-03 (Protocol ID: IMMU-132-11): A phase 2, open-label study of sacituzumab govitecan (IMMU-132) in subjects with metastatic solid tumours. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Sacituzumab Govitecan (Anti-HRS7-SN38 ADC) |
Dr. Ka Yu Tse |
Department of Obstetrics & Gynaecology |
tseky@hku.hk |
Dr. Ka Yu Tse
2255 5102 9061 9609 |
|
4.0 Lung |
||||||
|
4.2.1 Supportive Cancer Care |
||||||
|
Early Stage (Resectable) |
||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|
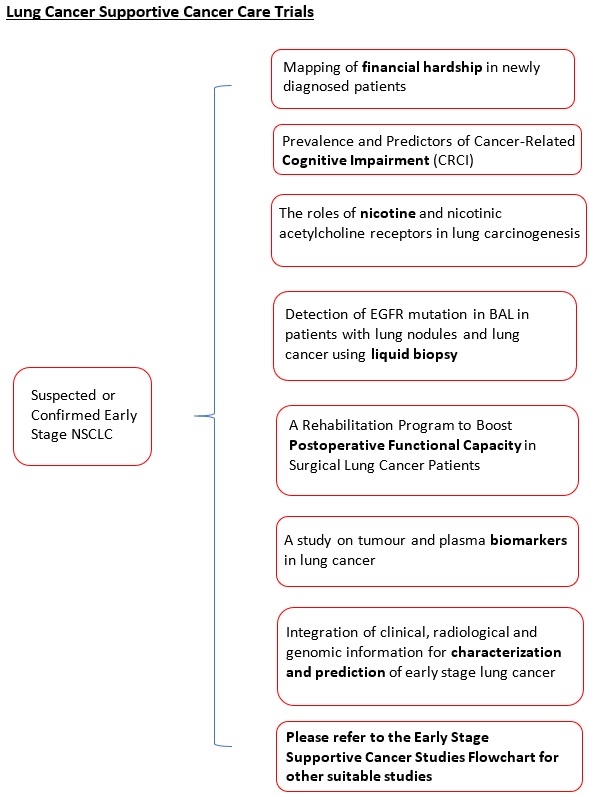
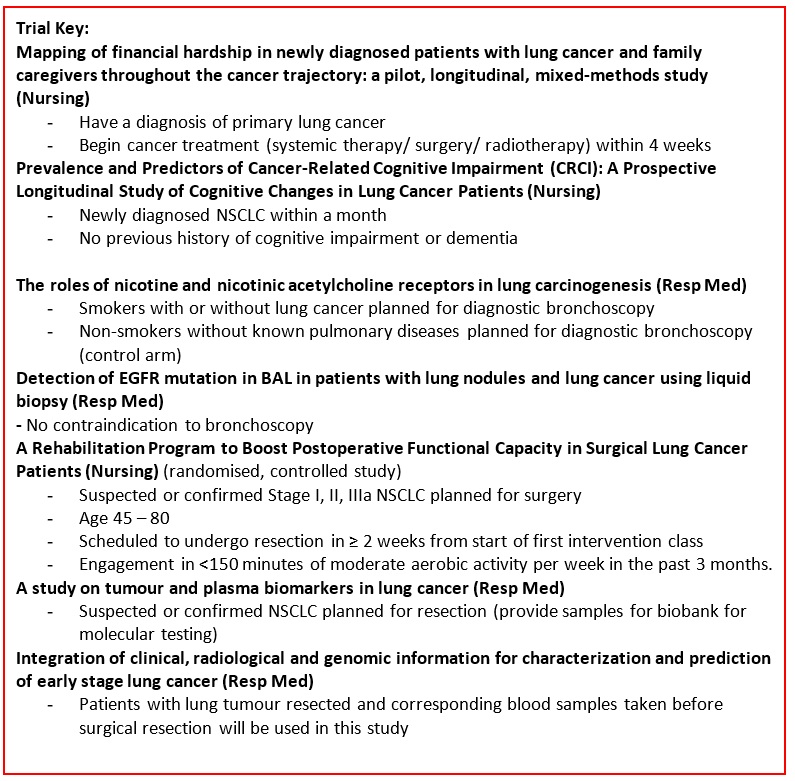
A rehabilitation Program to Boost Postoperative Functional Capacity in Surgical Lung Cancer Patients: A Randomized Controlled Trial. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Prof. Chia-Chin Lin |
School of Nursing |
cclin@hku.hk |
3917 6633 |
|
|
4.2.2 Supportive Cancer Care |
||||||
|
Advanced or Metastatic |
||||||
|
Effect of Tai-Chi versus Aerobic Exercise on Emotional Symptom Cluster in Late-stage Lung Cancer Patients: A Mixed-methods Intervention Evaluation with Mediation Analysis. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Prof. Chia-Chin Lin |
School of Nursing |
cclin@hku.hk |
3917 6633 |
|
|
|
4.0 Lung |
|||||
|
|
4.3.1 Data Science |
|||||
|
|
Specific Selection Criteria: Begin cancer treatment within four weeks |
|||||
|
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|
Mapping the complexities of financial hardship in dyads of patients with lung cancer and family caregivers throughout the cancer trajectory: a pilot, longitudinal, mixed-methods study |
Key Inclusion Criteria: Patients
Key Exclusion criteria: Patients
|
Prof. Chia-Chin Lin |
School of Nursing |
cclin@hku.hk |
3917 6633 |
|
|
4.3.2 Data Science (NSCLC) |
|||||
|
|
Specific Selection Criteria: Newly diagnosed with NSCLC within a month |
|||||
|
|
Prevalence and Predictors of Cancer-Related Cognitive Impairment (CRCI): A Prospective Longitudinal Study of Cognitive Changes in Lung Cancer Patients |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr Mu-Hsing Ho |
School of Nursing |
mhbho@hku.hk |
3910 2787 |
|
|
4.3.3 Data Science |
|||||
|
|
Epidemiology of lung cancer. |
Dr. C.L. Cheung |
Department of Pharmacology and Pharmacy |
lung1212@hku.hk |
3917 9462 |
|
|
|
4.3.4 Data Science (Cancer Screening) |
|||||
|
|
Specific Selection Criteria: Age 50 – 75, being first degree relatives with history of lung cancer |
|||||
|
|
Screening for lung cancer in subjects with family history of lung cancer. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.3.5 Data Science (Cancer Screening) |
|||||
|
|
Specific Selection Criteria: NOT previously diagnosed with lung cancer, NO suspicious symptoms, age 55-80, current or former smokers |
|||||
|
|
Prospective Evaluation of selection criteria for lung cancer screening with low-dose thoracic computed tomography and standardized system for nodule management - an international study. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.0 Lung |
|||||
|
|
4.4.1 Translational Research (NSCLC) |
|||||
|
|
Specific Selection Criteria: Resection done and additional clinical samples obtained and banked up |
|
|
|
|
|
|
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|
A study on tumor and plasma biomarkers in lung cancer. |
Key Inclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.2 Translational Research (NSCLC) |
|||||
|
|
Advanced |
|||||
|
|
Specific Selection Criteria: EGFR Del 19 or L858R Mutations, before First-line treatment |
|||||
|
|
Circulating tumor DNA (ctDNA) as a prognostic tool in patients with advanced lung adenocarcinoma. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.3 Translational Research (NSCLC) |
|||||
|
|
Advanced or Metastatic |
|||||
|
|
Specific Selection Criteria: EGFR Mutation with TKI treatment |
|||||
|
|
Establishment and application of molecular tests for personalized treatment of lung cancer. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.4 Translational Research |
|||||
|
|
Early Stage |
|||||
|
|
Specific Selection Criteria: Lung tumors resected |
|||||
|
|
Integration of clinical, radiological and genomic information for characterization and prediction of early stage lung cancer |
Key Inclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.5 Translational Research |
|||||
|
|
Specific Selection Criteria: Any one undergo diagnostic bronchoscopic examination |
|||||
|
|
The roles of nicotine and nicotinic acetylcholine receptors in lung carcinogenesis. |
Key Inclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.6 Translational Research |
|||||
|
|
Specific Selection Criteria: Lung cancer patients undergo bronchoscopy |
|||||
|
|
Detection of EGFR mutation in BAL in patients with lung nodules and lung cancer using liquid biopsy. |
Key Inclusion Criteria:
|
Dr. David CL Lam |
Department of Medicine |
dcllam@hku.hk |
2255 5814 |
|
|
4.4.7 Translational Research |
|||||
|
|
Specific Selection Criteria: Local residents in Xuan Wei using coal |
|||||
|
|
Do inflammatory processes induced by berthierine (Fe-Al serpentine) in coal play a role in the lung cancer epidemic of Xuan Wei, China? |
Key Inclusion Criteria:
|
Dr. Linwei Tian |
School of Public Health |
linweit@hku.hk |
39176351 |
|
4.0 Lung |
||||||
|
4.5.1 Basic Research (NSCLC) |
||||||
|
Study Title |
Principal Investigator |
Department |
|
Contact number |
||
|
A study to investigate the efficacy of ABT-199 targeting on lung cancer associated fibroblasts (CAFs) to inhibit regional lymph node metastasis on NSCLC orthotopic xenograft mouse model |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||
|
4.5.2 Basic Research (NSCLC) |
||||||
|
Identification of novel regulators of MHC class I upon IL-9 stimulation using CRISPR screening in non-small cell lung cancer (Madam Madeline Tong Lai Sheung Cancer Research Fund, School of Clinical Medicine, HKU) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||
|
4.5.3 Basic Research (NSCLC) |
||||||
|
Exploration of CRISPR/Cas9 gene editing (knockout) delivery system for treating EML4-ALK non-small cell lung cancer |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||
|
4.5.4 Basic Research (NSCLC) |
||||||
|
A study to investigate the efficacy of ABT-199 targeting on lung cancer associated fibroblasts (CAFs) to inhibit regional lymph node metastasis on NSCLC orthotopic xenograft mouse model |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||
|
4.5.5 Basic Research (NSCLC: Pharmacology) |
||||||
|
Feasibility of next-generation inhalable nanoagglomerated microparticles to improve the therapeutic outcomes of non-small cell lung cancer: A pilot study. |
Dr. Aviva Chow |
Department of Pharmacology and Pharmacy |
asfchow@hku.hk |
3917 9026 |
||
|
4.5.6 Basic Research (EGFR mutation) |
||||||
|
Exploiting synthetic lethal interactions with EGFR inhibitor for translation to cancer therapies |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
||
|
4.5.7 Basic Research (EGFR mutation) |
||||||
|
Elucidation of p38 MAPK signaling in mediating resistance to EGFR-TKIs |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
||
|
4.5.8 Basic Research |
||||||
|
Study of the therapeutic effect and underlying mechanism of anti-GITR and anti-PD-1 combination therapy in lung cancer treatment |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||
|
4.5.9 Basic Research |
||||||
|
Contribution of Late Permian C1 coal chamosite to lung cancer epidemic in Xuan Wei inferred by K-rasLA1 mice model” funded by National Natural Science Foundation of China (NSFC).
|
Dr. Linwei Tian |
School of Public Health |
linweit@hku.hk |
39176351 |
||
|
4.5.10 Basic Research (Mesothelioma) |
||||||
|
Identification of arsenic trioxide-resistant genes in cisplatin-resistant mesothelioma cells using CRISPR screening (Pneumoconiosis Compensation Fund Board, HK) |
Dr. James Chung Man Ho |
Department of Medicine |
jhocm@hku.hk |
2255 4349 |
||

Follow HKUMed