- 6.1. Clinical Trial
- 6.1.1. Colorectal
- 6.1.2. Rectal
- 6.1.3. Esophageal
- 6.1.4. Gastric
- 6.1.5. Pancreas
- 6.1.6. Other
- 6.1.6.1. Gastrointestinal malignancies from various GI sites
- 6.2. Supportive Cancer Care
- 6.3. Basic Research
|
6.0 Gastrointestinal |
||||||
|
6.1 Clinical Trial |
||||||
|
6.1.1.1 Colorectal |
||||||
|
Metastatic |
||||||
|
Second-line treatment (Progressed on or after 1 prior systemic therapy): Phase 2 |
Immunotherapy (Anti-CD47 Monoclonal Antibody), Targeted Therapy (Anti-VEGF Monoclonal Antibody), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
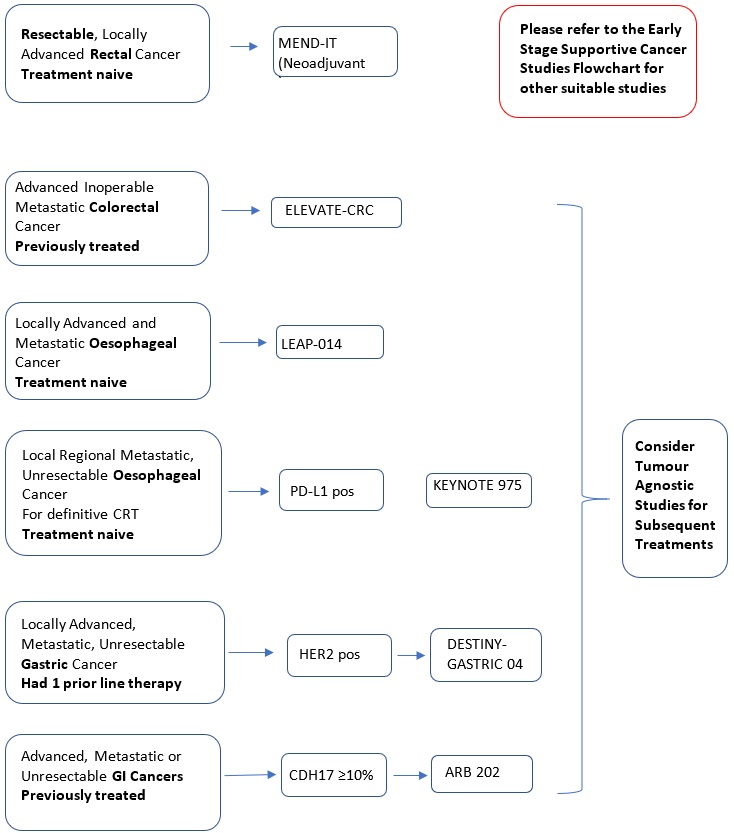
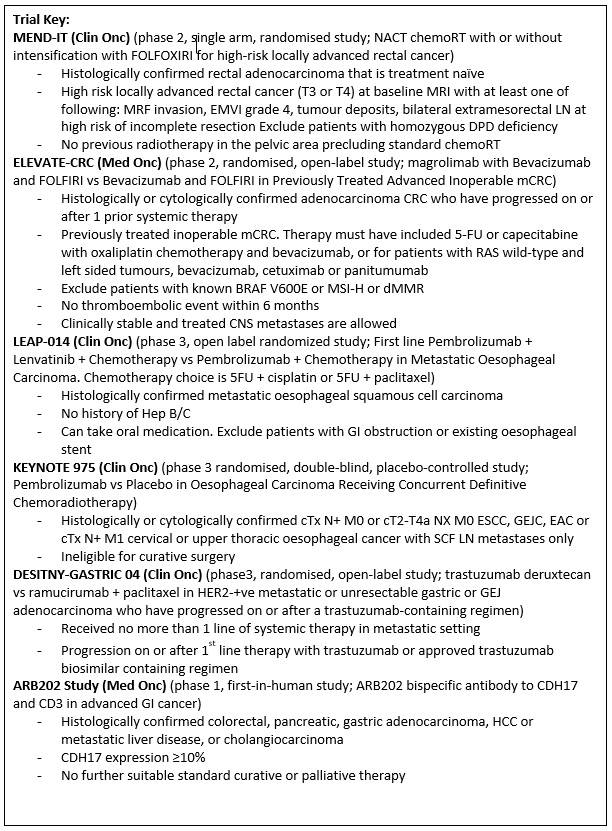
ELEVATE CRC (Protocol ID: GS-US-587-6156) A Phase 2, Randomized, Open-Label Study Evaluating the Safety and Efficacy of Magrolimab in Combination With Bevacizumab and FOLFIRI Versus Bevacizumab and FOLFIRI in Previously Treated Advanced Inoperable Metastatic Colorectal Cancer (mCRC) |
Key Inclusion Criteria:
|
Bevacizumab (Anti-VEGF Monoclonal Anitbody) + FOLFIRI +/- Magrolimab (Anti-CD47 Monoclonal Antibody) |
Dr. Thomas Yau |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
6.1 Clinical Trial |
||||||
|
6.1.2 Rectal |
||||||
|
Locally Advanced, Resectable |
||||||
|
Neoadjuvant treatment |
Concurrent Chemoradiotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
MEND-IT (Protocol ID: NL74465.100.20): A randomized study of neoadjuvant chemoradiotherapy with or without intensification with the FOLFOXIRI chemo-regimen for high-risk locally advanced rectal cancer. |
Key Inclusion Criteria:
|
Control: Concurrent neoadjuvant concurrent capecitabine-radiotherapy (Chemo) followed by surgery and post-operative chemotherapy.
Vs.
Experimental: Neoadjuvant FOLFOXIRI x 4 cycles then concurrent capecitabine-radiotherapy (Chemo), surgery and post-operative chemotherapy.
|
Dr. Aya El HELALI |
Department of Clinical Oncology
|
ahelali@hku.hk |
Stephen AU 2255 5034 |
|
6.1 Clinical Trial |
||||||
|
6.1.3.1 Esophageal |
||||||
|
Metastatic |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-1 antibody), Targeted therapy (VEGFR Inhibitor), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
LEAP-014 (Protocol ID: MK-7902-014): A Phase 3, Randomized Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) + Lenvatinib (E7080/MK-7902) + Chemotherapy Compared With Standard of Care as First-line Intervention in Participants With Metastatic Esophageal Carcinoma. |
Key Inclusion Criteria:
|
Pembrolizumab (Anti-PD-1 monoclonal antibody) + lenvatinib (VEGFR Inhibitor) + Chemo
Vs.
Pembrolizumab + Chemo
*investigator's choice of chemotherapy with FP IV or TP IV |
Prof. Dora KWONG |
Department of Clinical Oncology
|
dlwkwong@hku.hk |
Alex MAK 2255 4216 |
|
6.1.3.2 Esophageal |
||||||
|
Local Regional Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: PD-L1 positive |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-1), Chemotherapy |
|||||
|
KEYNOTE 975 (Protocol ID: MK-3475-975): A Randomized, Double-blind, Placebo-controlled Phase 3 Trial of Pembrolizumab (MK-3475) Versus Placebo in Participants With Esophageal Carcinoma Receiving Concurrent Definitive Chemoradiotherapy. |
Key Inclusion Criteria:
|
Pembrolizumab (Anti-PD-1 Monoclonal Antibody) + Chemoradiotherapy
Vs.
Placebo + Chemo |
Prof Dora KWONG |
Department of Clinical Oncology
|
dlwkwong@hku.hk |
Alex MAK 2255 4216 |
|
6.1 Clinical Trial |
||||||
|
6.1.4 Gastric: HER2+ve |
||||||
|
Locally Advanced or Metastatic, Unresectable |
||||||
|
Second-line treatment: Phase 3 |
Targeted therapy (Anti-HER2 ADC/ Anti-VEGFR), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
DESTINY-Gastric04 (Protocol ID: DS8201-A-U306) A Phase 3, multicenter, 2-arm randomized, open-label study of trastuzumab deruxtecan in subjects with HER2-positive metastatic and/or unresectable gastric or gastro-esophageal junction (GEJ) adenocarcinoma subjects who have progressed on or after a trastuzumab-containing regimen. |
Key Inclusion Criteria:
|
Trastuzumab Deruxtecan (Anti-HER-2 ADC)
Vs.
Ramucirumab (Anti-VEGFR) + Paclitaxel |
Dr Wendy Chan |
Department of Clinical Oncology
|
winglok@hku.hk |
Michael WONG 2255 4216 |
|
6.1 Clinical Trial |
||||||
|
6.1.6 Other |
||||||
|
6.1.6.1 Gastrointestinal malignancies from various GI sites |
||||||
|
Advanced or Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: ≥10% by expression of CDH17 after/or standard treatment |
||||||
|
Two plus-line treatment: Phase 1 |
Immunotherapy (Anti-CDH17/CD3 bispecific T-cell engager Monoclonal Antibody) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
A Phase 1, first-in-human study of ARB202, bispecific antibody to CDH17 and CD3 in advanced gastrointestinal malignancies. |
Key Inclusion Criteria:
|
ARB202 (Anti-CDH17/CD3 bispecific T-cell engager Monoclonal Antibody) |
Dr. Roland Leung |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
6.0 Gastrointestinal |
|||||
|
6.2.1 Supportive Cancer Care |
|||||
|
Early Stage |
|||||
|
Specific Selection Criteria: Completed hospital-based adjuvant treatments including radiotherapy and chemotherapy, within the past 18 months. |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
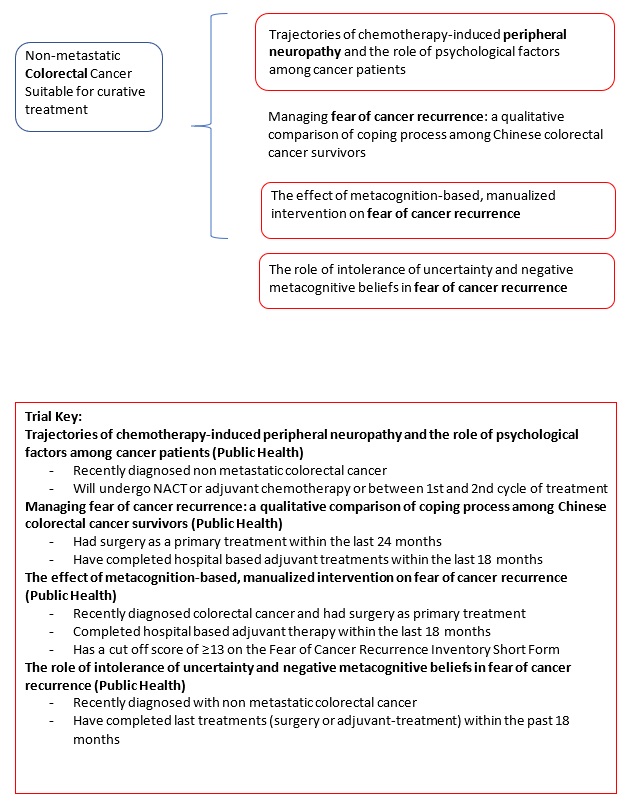
Trajectories of chemotherapy-induced peripheral neuropathy and the role of psychological factors among cancer patients. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Wendy WT Lam |
School of Public Health |
wwtlam@hku.hk |
3917 9878 |
|
6.2.2 Supportive Cancer Care |
|||||
|
Early Stage |
|||||
|
Specific Selection Criteria: Completed hospital-based adjuvant treatments including radiotherapy and chemotherapy, within the past 18 months. |
|||||
|
Managing fear of cancer recurrence: a qualitative comparison of coping process among Chinese breast cancer survivors with nonclinical, subclinical, and clinically-significant fear of cancer recurrence. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Danielle WL Ng |
School of Public Health |
dwlng@hku.hk |
3917 9897 |
|
6.2.3 Supportive Cancer Care |
|||||
|
Early Stage |
|||||
|
Specific Selection Criteria: Scheduled to commence their first cycle of outpatient adjuvant chemotherapy |
|||||
|
The effect of metacognition-based, manualized intervention on fear of cancer recurrence: a randomized controlled trial |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Wendy WT Lam |
School of Public Health |
wwtlam@hku.hk |
3917 9878 |
|
6.0 Gastrointestinal |
|||||
|
6.3.1 Basic Research |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
The role of intolerance of uncertainty and negative metacognitive beliefs in fear of cancer recurrence: a longitudinal study. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Wendy WT Lam |
School of Public Health |
wwtlam@hku.hk |
3917 9878 |
|
6.3.2 Basic Research |
|||||
|
Silica fragments in human esophagus tissue and its association with esophageal cancer: a case-control study |
Local residents in high- and low-incidence areas of China with different dietary cultures and environmental conditions (climate, soil, water). |
Dr. Linwei Tian |
School of Public Health |
linweit@hku.hk |
39176351 |
|
The glass roots of esophageal cancer in China: needle-shaped diatom frustules in trash fish |
|||||
|
6.3.3 Basic Research |
|||||
|
Study Title |
Principal Investigator |
Department |
|
Contact number |
|
|
Pharmacological effect of Amauroderma rugosum on colon cancer. |
Dr. George P.H. Leung |
Department of Pharmacology and Pharmacy |
gphleung@hku.hk |
3917 6861 |
|
|
6.3.4 Basic Research |
|||||
|
Nanostring nCounter expression profiling of endogenous transposable elements as a biomarker of prognosis in colorectal cancer |
Dr. Jason Wing Hon Wong |
School of Biomedical Sciences |
jwhwong@hku.hk |
Dr. Jason Wing Hon Wong 3917 9187 |
|
|
6.3.5 Basic Research |
|||||
|
Development of Photo-targeted Nanocarriers for Esophageal Cancer Therapy. |
Dr. W.P. Wang |
Department of Pharmacology and Pharmacy |
wangwp@hku.hk |
3917 9129 |
|
|
6.3.6 Basic Research |
|||||
|
Investigation of effects and molecular mechanisms of FGFR2-positive cancer-associate fibroblasts on tumor microenvironment in esophageal squamous cell carcinoma. |
Prof. Xin-yuan Guan |
School of Biomedical Sciences |
xyguan@hku.hk |
2255 4352 |
|
|
6.3.7 Basic Research |
|||||
|
Gastric Cancer Genomics and Beyond - Moving from Patient Samples to 3D Organoid Cultures for Integrative Genomics Analysis, Drug Sensitivity Assays, Cell Biological Studies and Animal Models. |
Prof. Suet Yi Leung |
Department of Pathology |
suetyi@hku.hk
|
2255 2664 |
|
|
6.3.8 Basic Research |
|||||
|
Targeted degradation of RhoA Y42C mutant protein by Proteolysis-targeting chimera (PROTAC) for gastric cancer therapy.
|
Dr Clive Yik Sham Chung |
School of Biomedical Sciences |
cyschung@hku.hk |
Dr Clive Yik Sham Chung 3917 9172 |
|
|
6.3.9 Basic Research |
|||||
|
Pancreatic Cancer Early Diagnosis using Aptamers |
Prof. Julian Alexander Tanner |
School of Biomedical Sciences |
jatanner@hku.hk |
Prof. Julian Alexander Tanner 3917 9472 |
|

Follow HKUMed