- 7.1. Clinical Trial
- 7.1.1. Kidney
- 7.1.2. Bladder
- 7.1.3. Prostate
|
7.0 Genitourinary |
||||||
|
7.1 Clinical Trial |
||||||
|
7.1.1 Kidney |
||||||
|
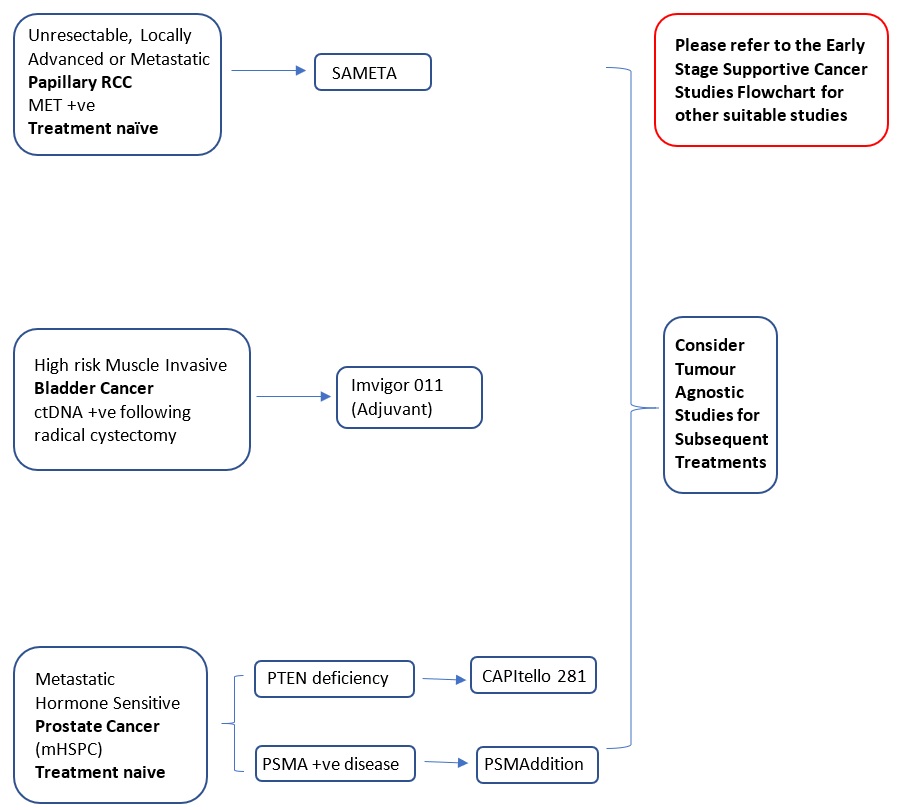
Locally Advanced or Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: MET+ve |
||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-L1 Antibody), Targeted Therapy (c-Met Receptor Inhibitor)/ (VEGFR Inhibitor) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
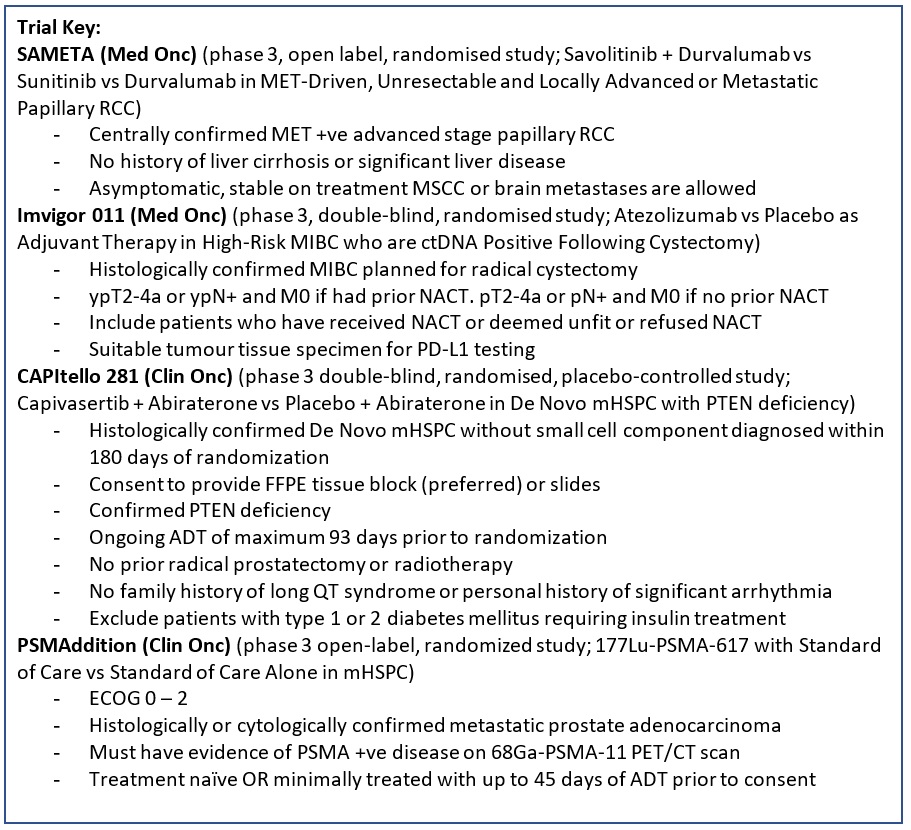
SAMETA (Protocol ID: D5086C00001): A Phase III, Open Label, Randomised, 3-Arm, Multi-Centre Study of Savolitinib Plus Durvalumab Versus Sunitinib and Durvalumab Monotherapy in MET-Driven, Unresectable and Locally Advanced or Metastatic Papillary Renal Cell Carcinoma. |
Key Inclusion Criteria:
|
Durvalumab (Anti-PD-L1 Monoclonal Antibody) + savolitinib (c-Met Receptor Inhibitor)
Vs.
Durvalumab
Vs.
Sunitinib (VEGFR Inhibitor) |
Dr. Bryan Li |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
7.1 Clinical Trial |
||||||
|
7.1.2 Bladder |
||||||
|
Early Stage Prior Neoadjuvant Therapy: ypT2-4a or ypN+ and M0 Not received Neoadjuvant Therapy: pT2-4a or pN+ and M0 |
||||||
|
Specific Selection Criteria: PD-L1 (≥1%), ctDNA+ve after cystectomy |
||||||
|
Adjuvant treatment: Phase 3 |
Immunotherapy: Anti-PD-L1 Monoclonal Antibody |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
IMvigor011 (Protocol ID: BO42843): A Phase III, Double-Blind, Multicenter, Randomized Study of Atezolizumab (Anti-PDL1 Antibody) Versus Placebo as Adjuvant Therapy in Patients With High-Risk Muscle-Invasive Bladder Cancer Who Are ctDNA Positive Following Cystectomy. |
Key Inclusion Criteria:
|
Atezolizumab (Anti-PD-L1 Monoclonal Antibody)
Vs.
Placebo
|
Dr. Bryan Li |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
7.1 Clinical Trial |
||||||
|
7.1.3.1 Prostate |
||||||
|
Metastatic |
||||||
|
Specific Selection Criteria: Hormone-Sensitive, PTEN deficiency |
||||||
|
First-line treatment: Phase 3 |
Targeted therapy (Akt inhibitor), Androgen inhibitor |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
CAPItello-281 (Protocol ID: D361BC00001): A Phase III Double-Blind, Randomized, Placebo-Controlled Study Assessing the Efficacy and Safety of Capivasertib + Abiraterone Versus Placebo + Abiraterone as Treatment for Patients with De Novo Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) Characterized by PTEN deficiency. |
Key Inclusion Criteria:
|
Capivasertib (Akt inhibitor) + Abiraterone (Androgen biosynthesis inhibitors)
Vs.
Placebo + Abiraterone |
Dr. Steven Wai Kwan Siu |
Department of Clinical Oncology |
oncology@hku.hk |
Cliff CHONG 2255 5102 |
|
7.1.3.2 Prostate |
||||||
|
Hormone-Sensitive, Metastatic |
||||||
|
Specific Selection Criteria: PSMA+ve |
||||||
|
First-line treatment: Phase 3 |
Radioconjugate |
|||||
|
PSMAddition (Protocol ID: CAAA617C12301): An Open-label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With Standard of Care, Versus Standard of Care Alone, in Adult Male Patients With Metastatic Hormone Sensitive Prostate Cancer (mHSPC). |
Key Inclusion Criteria:
|
177 Lu-PSMA-617 (Radioconjugate) + SOC
Vs.
SOC |
Dr. Steven Wai Kwan Siu |
Department of Clinical Oncology |
oncology@hku.hk |
Cliff CHONG 2255 5102 |

Follow HKUMed