01 March 2021
Event Date(s)/Period(s)
March 2021
Organised by
HKU Phase 1 Clinical Trials Centre
Clinical Trials Centre (HKU-CTC)
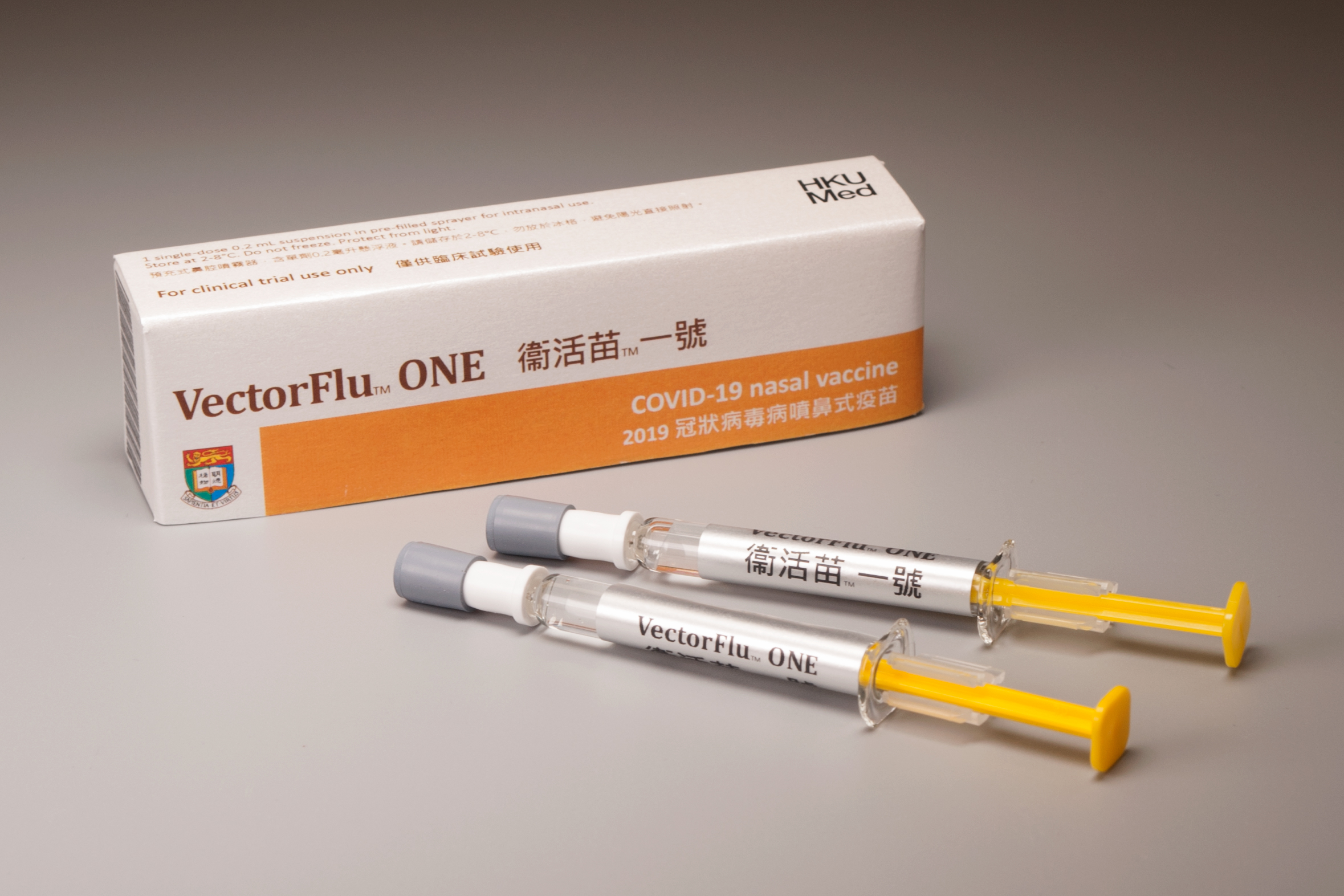
Phase 1 clinical trials of VectorFlu™ ONE, the world's first COVID-19 nasal vaccine, developed by the research team of HKU Department of Microbiology, commenced in March 2021 at the HKU Phase 1 Clinical Trials Centre.
VectorFlu™ ONE is the first novel vaccine developed in Hong Kong to go into human trials.. This vaccine strategy has been selected as one of the five vaccine technologies by the Ministry of Science and Technology of China for further evaluation. This phase 1 clinical trial is supported by the Health and Medical Research Fund of Hong Kong (HMRF) and the Coalition for Epidemic Preparedness Innovations (CEPI). The trial is run in accordance with the highest international ethical, regulatory, and industrial standards.
The successful initiation of this trial is an excellent example of multi-disciplinary collaboration, having involved the Department of Microbiology, Department of Medicine, Clinical Trials Centre, and Biostatistics and Clinical Research Methodology Unit.

Follow HKUMed