27 August 2024
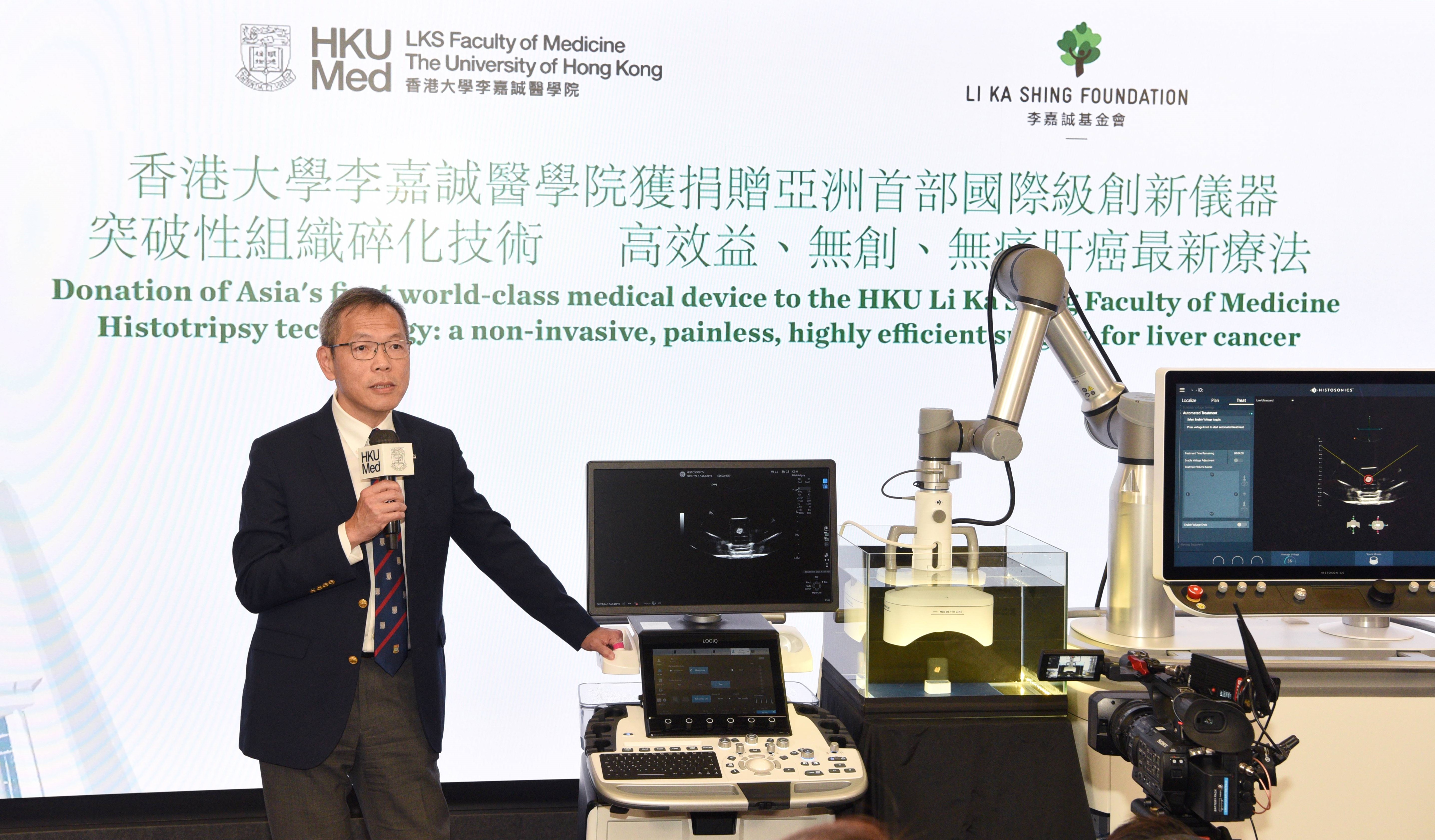
With a generous donation from the Li Ka Shing Foundation, the LKS Faculty of Medicine at the University of Hong Kong (HKUMed) will be the first institution in Asia to introduce the groundbreaking Histotripsy system, using state-of-the-art histotripsy technology for clinical trials, offering Hong Kong liver cancer patients access to non-invasive, painless and highly effective treatment.
Ten patients will be sponsored by the Foundation to receive this novel treatment as part of the initial clinical trials to be conducted at Queen Mary Hospital. The first patient is expected to undergo treatment within this week. The donation also supported six doctors and radiologists to attend specialised training at the US manufacturer HistoSonics to ensure they are comprehensively versed in the optimal utilisation of this cutting-edge technology.
Philanthropy empowers healthcare advancements
Professor Chak-sing Lau, Dean of Medicine, HKU, said he was deeply grateful for the generosity of the Foundation, as well as for its staunch support over the years, including a HK$30 million donation last year to ramp up the use of AI in teaching and learning. ‘This novel liver cancer treatment technology has gained popularity among surgeons and oncologists around the globe,’ he said. ‘We are very honoured to be the first institution in Asia, after the US and the UAE, to offer this treatment option to local patients, affirming Hong Kong's leading position in the quick adoption of cutting-edge medical technology.’
‘To build on this momentum, we are excited to establish the Liver Cancer Research Division in our Centre for Cancer Medicine, the first of its kind in Hong Kong, with a matching fund of not less than HK$10 million from the Faculty,’ continued Professor Lau. ‘This will allow us to assemble a multidisciplinary team of experts to collaborate on developing new treatment options for liver cancer. By forming the first histotripsy training and research hub outside the US, we are poised to step up research efforts in this promising field, expanding the application of this technology to a wider range of cancer types and ultimately benefiting more patients.’
Mr Li Ka-shing stated, ‘Killing cancer with bubbles is such a transformative technology. Advancing innovative medical research and its applications to ignite hope and spur economic growth in our society is critical to our future. I am grateful to University of Michigan for facilitating early access to HistoSonic's Histotripsy system to both of our medical schools so that painless, non-invasive, effective and affordable treatments can be provided to liver cancer patients in Hong Kong immediately. I eagerly await in fascination, the powerful benefits of histotripsy treatments to be extended beyond cancer to tackle other conditions, as it not only shines bright as hope to patients, but also a conduit to reduce the increasing burden of our medical budget.’
The second Histotripsy system donated to the Chinese University of Hong Kong's Faculty of Medicine is expected to arrive by the end of the year. Li Ka Shing Foundation will provide support to 20 patients treated at both universities .
Histotripsy: A paradigm shift in liver cancer treatment
Histotripsy is a promising non-invasive technology that uses high-intensity ultrasound waves to precisely disrupt cancer tissue without damaging the surrounding healthy tissue. This novel proprietary technology works by using targeted ultrasound waves to form microbubbles within the tumour. The bubbles rapidly expand and collapse, generating shock waves that disrupt and liquefy the targeted tumour cells, which is entirely different from high intensity focused ultrasound (HIFU) thermal therapy, which uses ultrasound to heat the target tissue.
The procedure involves no incisions or radiation, thus offering a painless, scarless and bloodless experience with no risk of infection or metastasis, providing a more targeted and effective treatment for liver cancer than traditional ablation, radiotherapy or surgery.
Since its FDA approval in October 2023, nearly 400 patients have been treated in US medical institutions, with significant efficacy, marking a major technological breakthrough in cancer treatment. Clinical data shows that patients who received the treatment recovered well post-operation, with no local recurrence of the target tumours and no major complications related to the treatment, confirming the safety and efficacy of the technology.
In addition, recent studies have revealed that histotripsy treatments may stimulate an immune response from the body leading to regression of untreated tumour regions via the abscopal response, paving the way to enhance therapeutic efficacy of combing histotripsy with immunotherapy in the future.
Addressing the unmet need for liver cancer treatment
Liver cancer is the fifth commonest cancer in Hong Kong, with approximately 1,800 new cases and over 1,500 deaths annually. Over 80% of cases are diagnosed at a late stage. Current treatments include surgical resection, liver transplantation, ablation, radiotherapy, chemotherapy, targeted therapy and immunotherapy, all of which require significant recovery time.
Professor Albert Chan Chi-yan, Clinical Professor, Department of Surgery, School of Clinical Medicine at HKUMed, said he is excited about the introduction of this pioneering technology. ‘Surgical resection is the gold standard for treating liver tumours, but not all patients are candidates for surgery owing to the location or size of the tumour or because of underlying liver disease. In these cases, non-invasive histotripsy may offer an alternative to surgical resection because of its promising non-invasive treatment of liver tumours.’
‘I am very honoured to lead the initial clinical trials in Hong Kong,’ said Professor Chan. ‘We expect histotripsy to be a safe and effective treatment option for liver tumours in the local patient population and the treatment to result in a significant reduction in tumour size and volume, with minimal adverse effects.’
Enquiry for the clinical trials: 2255 6646
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).




Follow HKUMed