HKU Discovers a New Immune Therapeutic Strategy for Controlling Epstein-Barr virus-induced tumor
17 Sep 2014
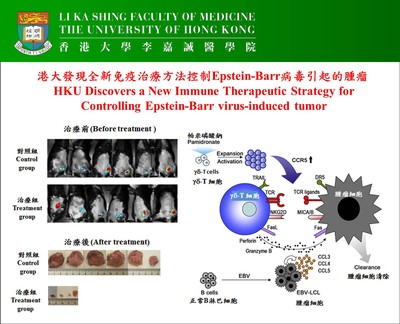
Epstein-Barr virus (EBV)-induced tumor, such as EBV-induced B cell lymphoproliferative disorders (EBV-LPD), is a serious and life-threatening condition with significant morbidity and mortality in immunocompromised patients. Currently, there are only very limited treatment options. An international research team, led by Dr Tu Wenwei, Associate Professor in Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU), has discovered that pamidronate, a drug well-established for treatment of bone diseases, can effectively control EBV-LPD by enhancing human gamma-delta T cell (γδ-T cell) immunity. The discovery has been published recently in the prestigious international scientific journal – Cancer Cell.
Research Implications
Dr Tu Wenwei, Associate Professor in Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, HKU, says, “The discovery has provided the proof-of-principle for a novel immune therapeutic approach using pamidronate to control EBV-induced tumors through boosting human γδ-T cell immunity in humanised mouse model and the result will be further tested on human for clinical trial”. Another researcher of the study, Professor Godfrey Chan Chi-fung, Tsao Yen-Chow Professor in Paediatrics and Adolescent Medicine, Clinical Professor and Head of Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, HKU says, “This new immune therapeutic approach may significantly reduce the unwanted side-effects and general immunosuppression caused by currently available treatment options”. Another author of the discovery, Professor Lau Yu-lung, Doris Zimmern Professor in Community Child Health and Chair Professor from the same department adds, “This new application of an ‘old drug’ potentially offers a safe and readily available option for the treatment of EBV-induced tumors because pamidronate has been already used for decades in osteoporosis treatment”.
Background of the study
Epstein-Barr virus (EBV) is a herpesvirus that latently infects human B cells in most individuals by adulthood. Persistent EBV infection is generally subclinical in immunocompetent hosts. However, immunocompromised patients are at high risk of developing EBV-induced tumors, such as EBV-induced B cell lymphoproliferative disorders (EBV-LPD), with significant morbidity and mortality. EBV-LPD in immunocompromised patients is a serious and life-threatening condition with limited treatment options. Current treatment options for EBV-LPD include restoring EBV-specific cytotoxic T lymphocytes (CTL) and depleting the B cells with monoclonal antibodies or chemotherapy. However, restoration of EBV-specific CTL is limited by the difficulties in generating enough numbers of EBV-specific CTL in vitro and the lack of in vivo expansion of infused CTL. Antibody-mediated targeting EBV-infected B cells and chemotherapy have unwanted side-effects and lead to general immunosuppression. Thus, there is an urgent need for developing better strategies to control EBV-LPD.
Research methods and findings
In current study, the team established a lethal EBV-LPD model with characteristics close to the human disease in humanised mice, in which the mice contain the complete human immune system. Through this humanised mouse model, HKU researchers find that pamidronate, an old drug for treatment of osteoporosis, can effectively prevent EBV-LPD development and induce EBV-induced tumor regression in humanised mice through boosting the immunity of human γδ-T cells. All control mice developed solid tumors, and 9 of 11 control mice died within 60 days. In contrast, only 2 of 10 mice developed solid tumors, and 9 of 10 mice survived for more than 100 days in treatment group. This research is at pre-clinical stage and now will be expanded to human clinical trials.
About the research team
The research team is led by Dr Tu Wenwei, Associate Professor in Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, HKU. Mr Zheng Xiang and Dr Liu Yinping from Department of Paediatrics & Adolescent Medicine are the co-first authors. Other researchers include Professor Godfrey Chan Chi-fung, Tsao Yen-Chow Professor in Paediatrics and Adolescent Medicine, Clinical Professor and Head of Department of Paediatrics & Adolescent Medicine, and Professor Lau Yu-lung, Doris Zimmern Professor in Community Child Health and Chair Professor of Paediatrics, Dr Zheng Jian, Miss Lv Aizheng, Mr Gao Yulong, Mr Lam Kowk-tai Lam from Department of Paediatrics & Adolescent Medicine, Professor George Tsao Sai-wah, Professor and Head of Department of Anatomy, Dr Chen Honglin, Associate Professor of Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU. International and mainland China collaborators include Professor Marc Bonneville from Université de Nantes, France, Dr Hu Huaidong from Chongqing Medical University and Dr Yang Yuanzhong from Sun Yat-Sen University, China.
This study was supported in part by General Research Fund, Research Grants Council of Hong Kong (HKU 781211M), the Area of Excellence program supported by the University Grants Committee of the Hong Kong SAR, China (AoE/M-12/06 and AoE/M-06/08).
To use the press release photo(s) for any publishing, publicity and related purpose, photo courtesy should be given to “Li Ka Shing Faculty of Medicine, The University of Hong Kong”

Group photo of the research team at the Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, HKU. (First row from left to right) Professor Lau Yu-lung, Doris Zimmern Professor in Community Child Health and Chair Professor of Paediatrics; Dr Tu Wenwei, Associate Professor; Professor Godfrey Chan Chi-fung, Tsao Yen-Chow Professor in Paediatrics and Adolescent Medicine, Clinical Professor and Head of the Department. (Second row from left to right) Dr Liu Yinping, Dr Zheng Jian and Mr Zheng Xiang.
HKU discovers a new immune therapeutic strategy for controlling Epstein-Barr virus-induced tumor.
