HKU scientists identify the genesis and source of avian influenza H7N9 virus causing human infections
Study published in prestigious international scientific journal, Nature
22 Aug 2013
An international research team, led by Professor Yi Guan and Dr Zhu Huachen of the State Key Laboratory of Emerging Infectious Diseases and the School of Public Health, The University of Hong Kong (HKU) Li Ka Shing Faculty of Medicine, in collaboration with Shantou University, St Jude Children’s Research Hospital, USA and UK scientists, identify the genesis and source of human infections of the avian influenza A (H7N9) virus recently isolated from humans in China. The researchers also find a previously unrecognised H7N7 avian virus and believe that H7N9 is not the only virus that has pandemic potential. This important research has been published this morning in the most prestigious international scientific journal, Nature.
Key research findings
Professor Yi Guan, Daniel CK Yu Professor in Virology, Professor of the School of Public Health HKU Li Ka Shing Faculty of Medicine says, “This study shows that chickens at live-poultry markets are very likely the source of H7N9 human infections. The virus was predominantly isolated from oropharyngeal swabs taken from these birds. At the time of the H7N9 outbreak, there was a previously unrecognised, but related, H7N7 lineage which also emerged in chickens in the live poultry markets of east China. Under experimental conditions, these H7N7 viruses, like the H7N9 viruses, have the ability to infect and cause pneumonia in ferrets, the most accepted animal model for research into human influenza.”
Dr Zhu Huachen, Research Assistant Professor of the School of Public Health points out, “H7 viruses probably transferred from ducks to chickens on at least two independent occasions. Domestic ducks can pass viruses or virus genes that originally came from wild birds on to chickens. Further reassortment with the currently prevalent H9N2 viruses in chickens generated the H7N9 outbreak lineage and the H7N7 viruses which can infect ferrets and potentially humans.”
The researchers identified at the live poultry markets of east China that the H7N7 viruses are highly related to the human isolated H7N9 viruses and seven of their gene segments have common ancestors, but their NA genes belong to different subtypes. This novel H7N7 virus also emerged in chickens at almost the same time as the H7N9 virus.
Experiments using the ferret animal model, which has a disease profile and transmission route for influenza resembling that of infected humans, revealed that this newly reported H7N7 virus also has the ability to infect ferrets and cause significant pneumonia. Infected ferrets shed virus via nasal and faecal routes, but majorly from the respiratory tract.
Research implications and suggestions
The avian H7N9 and newly recognised H7N7 viruses were predominantly isolated from market chickens, indicating that chickens at the live poultry markets are very likely the most important source of human infections. The finding that H7N9 viruses were majorly detected from the oropharyngeal samples of chickens suggests a preference for viral replication and shedding from the respiratory and oral tracts. The researchers suggest that a change in the poultry trading and marketing systems to prevent direct contact of human populations with live poultry, especial chickens, will be key to averting avian-to-human transmission of viruses.
Domestic ducks can receive and maintain viruses from wild birds; reassort those viruses and pass them on to chickens to generate novel variants with the potential to infect humans. Therefore, segregation of different bird species can prevent interspecies transmissions and subsequent reassortment events, contributing to the control of virus genesis and dissemination.
The identification of the novel H7N7 avian influenza viruses raises concerns that pandemic threats may go beyond the H7N9 virus. Before the closure of live poultry markets and the culling of poultry in the eastern China in April, there were at least two different reassortant H7 viruses prevailing in this area. The possibility that the H7N7 and H7N9 viruses can re-emerge cannot be excluded.
Research method
Following the H7N9 outbreak, the researchers collected samples from the oropharynx and digestive tracts of chickens, ducks, geese, pigeons, quail and wild birds in three Chinese provinces and Hong Kong. They isolated many influenza viruses and genetically sequenced those of the H7N9 subtype and related H7N7 and H9N2 viruses, along with H7 and N9 viruses from their long-term surveillance in China. From these data they were able to use advanced phylogenetic methods to reconstruct how the H7N9 virus evolved through various species of birds and to determine the origin of the genes. They also used ferrets, the well accepted animal model for human influenza research, to prove that this newly recognized H7N7 variant can infect and cause disease in ferrets.
Research Background
To date, a total of 135 laboratory-confirmed cases of human infection with avian influenza A (H7N9) virus have been reported in Mainland China and Taiwan since March this year. 45 deaths from A (H7N9) infection have been recorded.
About the research team
This research was led by Professor Yi Guan, Daniel C K Yu Professor in Virology, Professor of the School of Public Health and Dr. Zhu Huachen, Research Assistant Professor of the School of Public Health, The University of Hong Kong Li Ka Shing Faculty of Medicine, with contributions from a group of researchers from the HKU State Key Laboratory of Emerging Infectious Diseases, Shantou University Medical College, St Jude Children’s Research Hospital, USA, Oxford University and Edinburgh University, UK. The HKU influenza research team continues to make contributions to combat emerging infectious diseases.
To use the press release photo(s) for any publishing, publicity and related purpose, photo courtesy should be given to “Li Ka Shing Faculty of Medicine, The University of Hong Kong”
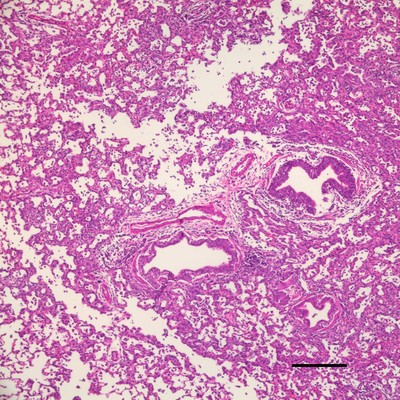
Other than H7N9, the researchers discovered a previously unrecognized, but related, H7N7 lineage at the poultry market of east China region. They are infectious to ferrets models, a most accepted animal model for research into human influenza and lead to pneumonia. This graph shows the lung of the animal model infected by avian influenza virus.
The international team led by Professor Yi Guan (middle), Daniel CK Yu Professor in Virology, Professor of School of Public Health and Dr Zhu Huachen (left), Research Assistant Professor of School of Public Health, identifies genesis and the source of human infections of the avian influenza A (H7N9) virus. The report written by Dr Tommy Lam Tsan-yuk (right), Research Assistant Professor, has been published this morning in the most prestigious international scientific journal, Nature.
